Highlights
The strength of the magnetic field is one of the most important properties of an NMR spectrometer. The dispersion (i.e. the “distance” of two peaks in an NMR spectrum) is enhanced at higher magnetic fields. For the investigation of substances with a large number of peaks, higher magnetic fields render it possible to separate different peaks from one another, making GHz-class NMR an invaluable tool for structure determination.
Another great advantage of higher magnetic fields is the improved sensitivity that can be achieved in an NMR experiment. Higher magnetic fields lead to a larger number of nuclear spins of the sample residing in the lower energy quantum state, which results in a stronger NMR signal. This is particularly beneficial for multi-dimensional NMR experiments, where the sensitivity increases additionally with the power of the number of dimensions.
For many years, high-resolution NMR was limited to a magnetic field of 23.5 Tesla, equivalent to a proton resonance frequency of 1.0 GHz. This limit was set by the physical properties of metallic, low-temperature superconductors (LTS), and it was first reached in 2009 with an Avance 1000 spectrometer at the Ultra-High Field NMR Center in Lyon, France.
High-temperature superconductors (HTS), first discovered in the 1980s, opened the door towards even higher magnetic fields at low temperatures, but considerable challenges in YBCO HTS tape manufacturing and in superconducting magnet technology made further UHF progress daunting until the early 2020ies.
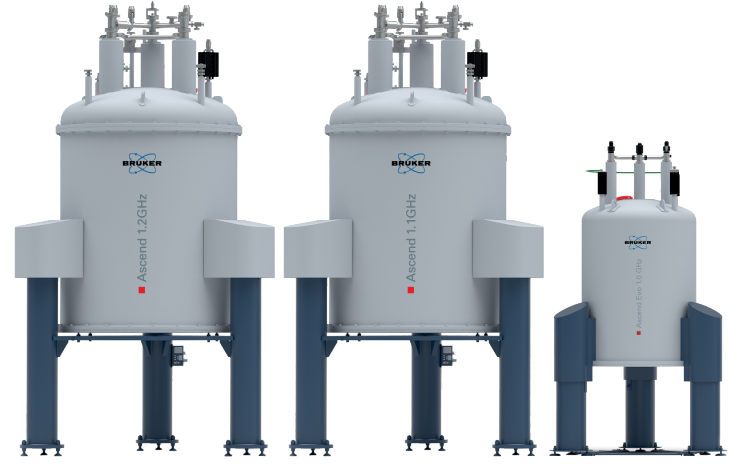
Bruker's 1.0 GHz Ascend Evo, and 1.1 and 1.2 GHz Ascend magnets utilize a sophisticated hybrid design with high-temperature superconductor (HTS) in the inner sections and low-temperature superconductors (LTS) in the outer sections of the magnet, as illustrated in Figure 2. Bruker’s GHz-class NMR magnets feature a 54 mm room-temperature bore (“standard bore”) and have exquisite homogeneity and field stability compatible with the demanding requirements of high-resolution NMR.
Benefits
The enhanced resolution and sensitivity make GHz-class NMR the ideal tool for many areas of research, in particular for material science and structural biology. The most important benefits are the following:
- Less sample requirement: Due to the enhanced sensitivity, UHF NMR typically requires only small amounts of sample, which is particularly advantageous for rare and limited samples.
- Atomic-level resolution: GHz-class NMR provides atomic-level resolution on an unprecedented level. For biological samples, this is particularly pronounced for small and medium-sized proteins.
- Solution-state information: GHz-class NMR can work in solution, offering insights into the behavior of biomolecules in conditions closer to their native environment, which is essential for understanding their biological functions.
- Analysis of dynamic structures: GHz-class NMR spectroscopy is excellent for studying the dynamics and motions of biomolecules in solution, providing insights into conformational changes, flexibility, and interactions. This capability to study dynamics is particularly beneficial in understanding protein folding, function, and interactions.
- Ability to study ligand interactions: UHF NMR is well-suited for studying interactions between proteins and small molecules, enabling detailed analysis of binding sites and dynamics, crucial for drug discovery and design.
-

-

-

-
-

-

-

-
-

-

-

-
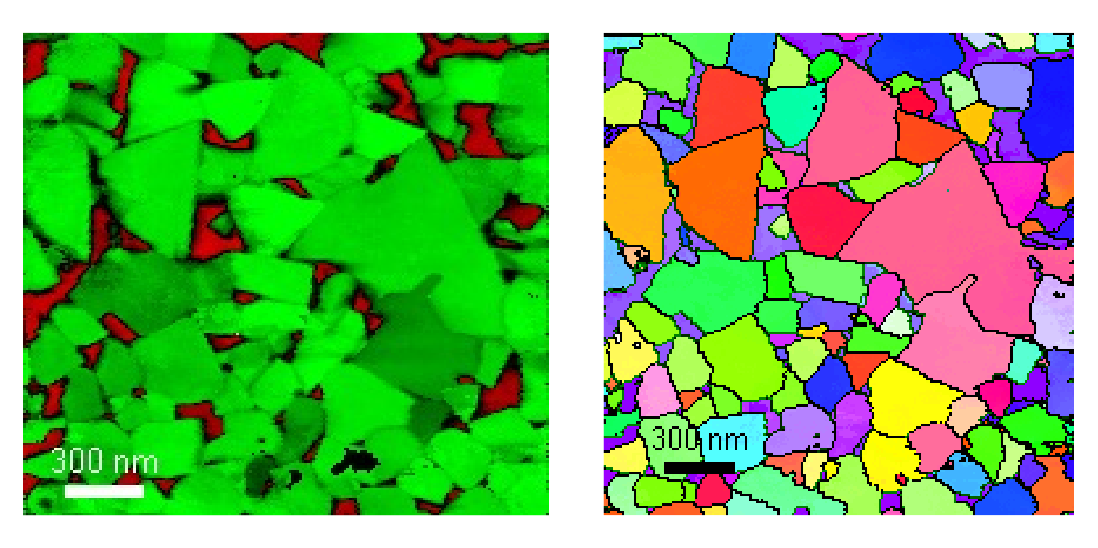
-
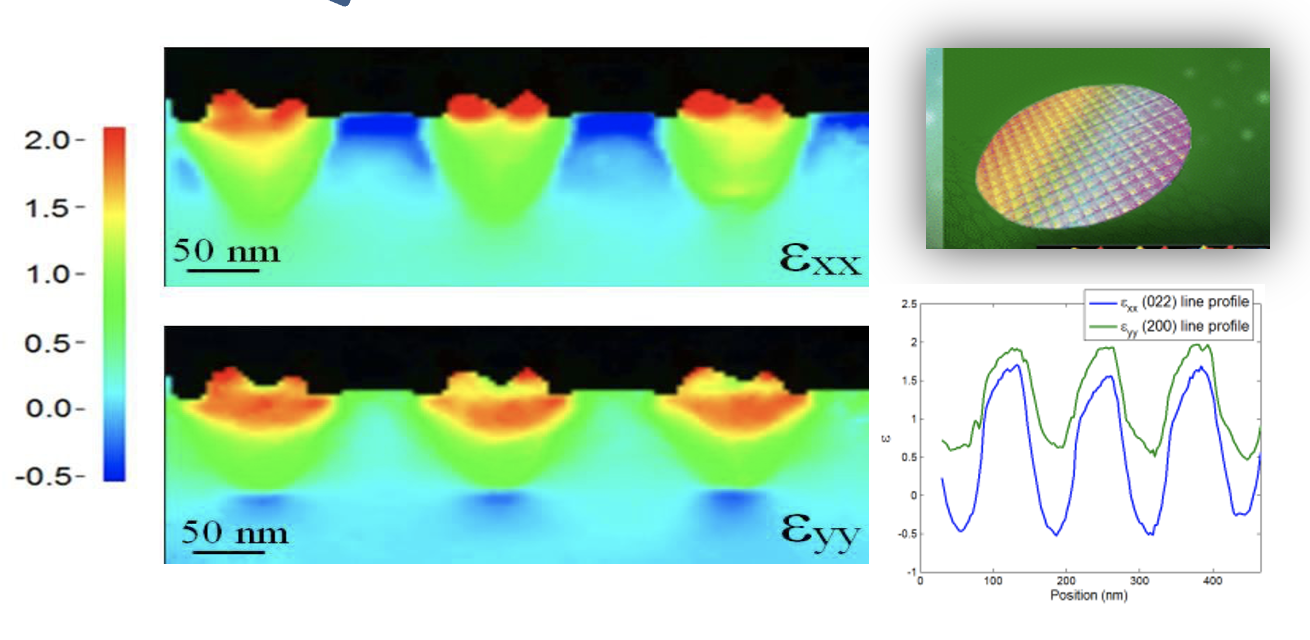
-

-
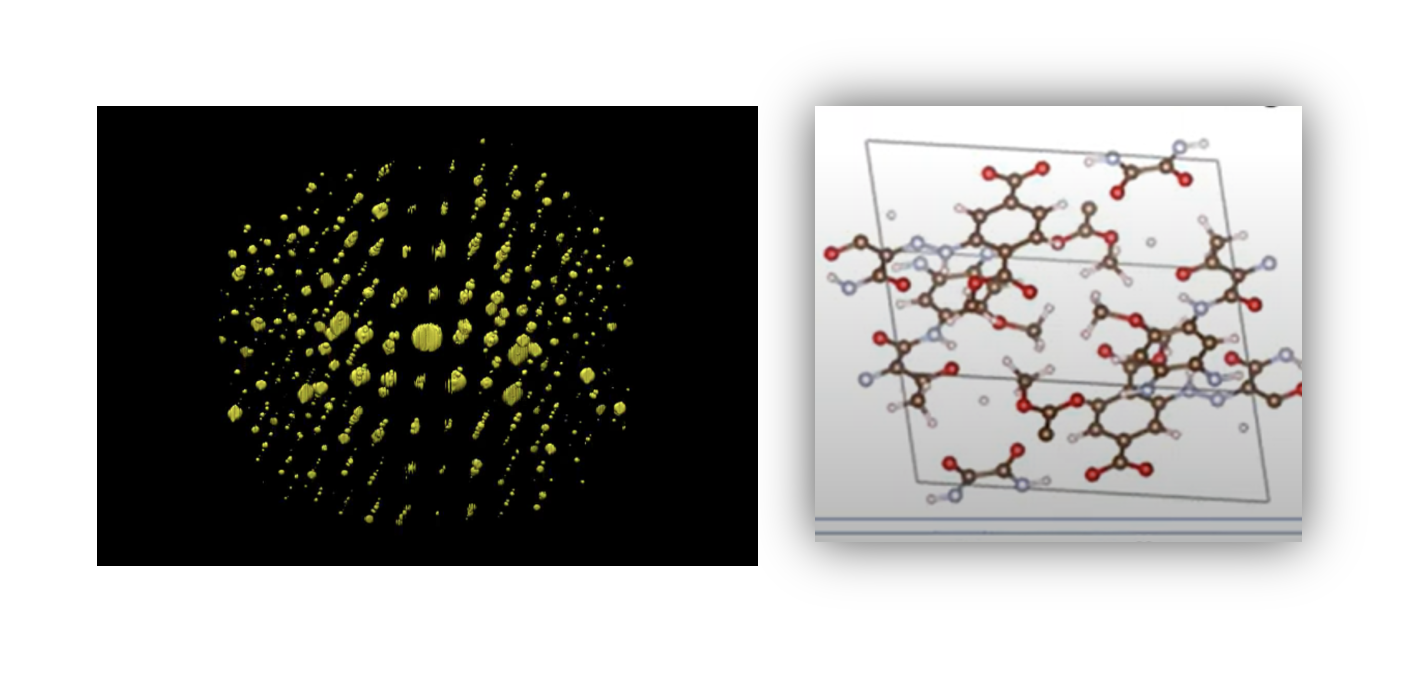
-

-
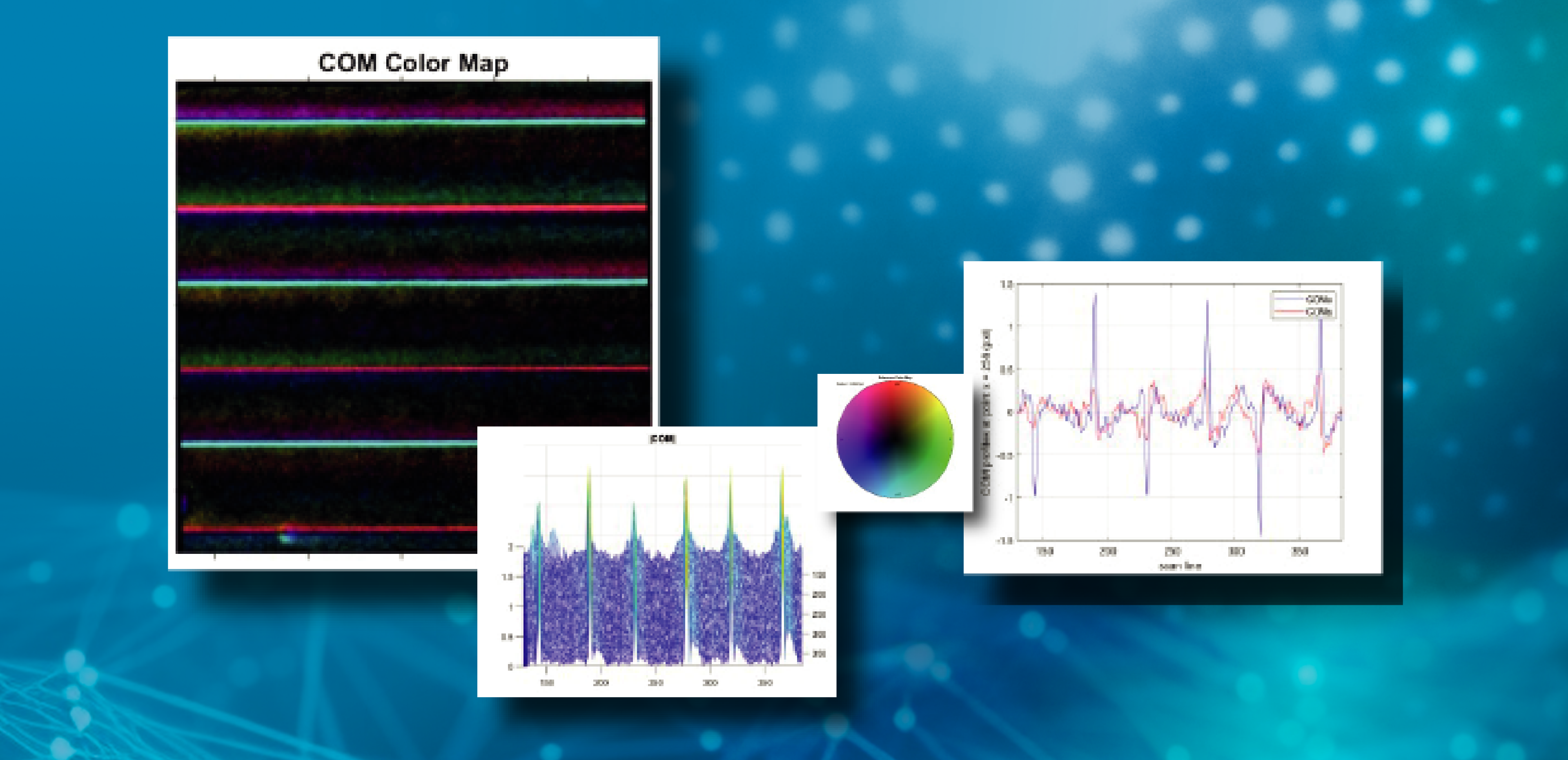
-
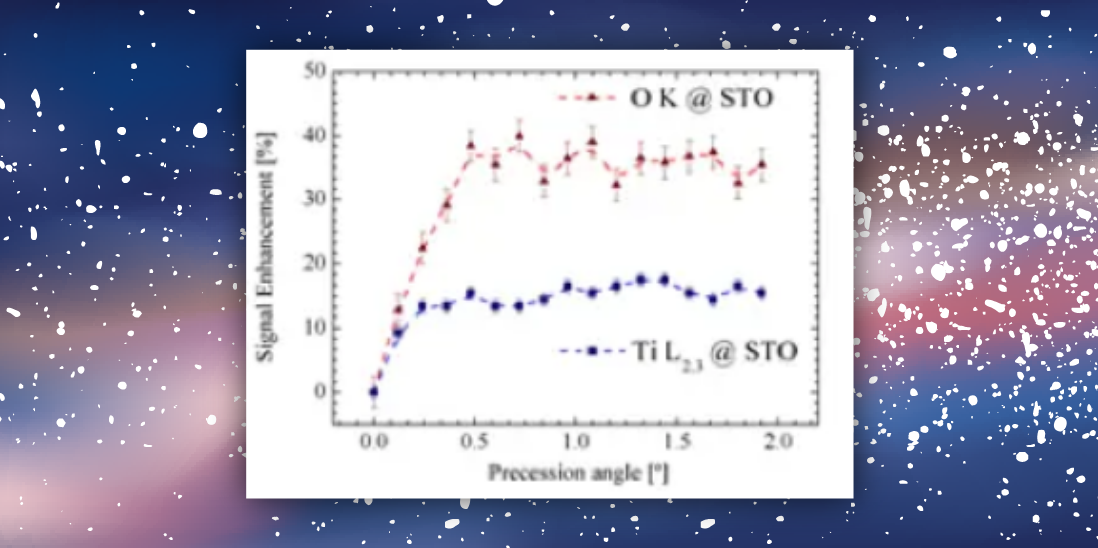
-

-

-
-
-

-

-

-

-

-

-

-
-
-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-
-
-

-

-

-

-

-

-

-

-
-

-

-
-
-

-

-